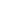
Register Trials / WHO data set
The minimum amount of trial information that must appear in a register in order for a given trial to be considered fully registered. There are currently 24 items in the WHO Trial Registration Data Set (TRDS).
As a primary registry of WHO ICTRP, ITMCTR strictly adheres to the TRDS to maintain transparency and standardization of global clinical trial information.
WHO trial registration data set:
No.
Items
1
Items
2
Date of registration in primary registry
3
Secondary identifying numbers
4
Sources of monetary or material support
5
Primary sponsor
6
Secondary sponsors
7
Contact for public queries
8
Contact for scientific queries
9
Public title
10
Scientific title
11
Countries of recruitment
12
Health conditions or problems studied
13
Interventions
14
Key inclusion and exclusion criteria
15
Study type
16
Date of first enrollment
17
Target sample size
18
Recruitment status
19
Primary outcomes
20
Key secondary outcomes
21
Ethics review
22
Completion date
23
Sharing statement
24
IPD sharing statement
See the explanation to each item. https://www.who.int/clinical-trials-registry-platform/network/who-data-set